39 what is the term used to label the energy levels of electrons
quizlet.com › 164693580 › chemistry-ch5-flash-cardsChemistry Ch.5 Flashcards | Quizlet The electrons in an atom can exist between energy levels energy levels What are the fixed energies of electrons called move an electron from its present energy level to a higher one. A quantum of energy is the amount of energy required to further In general, the higher the electron is on the energy ladder, the_________ it is from the nucleus. true what is the term used to label the energy levels of electrons Web The maximum number of electrons at a given energy level depends on its number of orbitals. The electrons of an atom tend to fill all the lowest energy levels first and the higher ones after that. Web what is the term used to label the energy levels of electrons. Web What is the term used to label the energy levels of electrons.
What term is used to label the energy levels of electrons? The term that is used to label the energy levels of electrons are principle quantum numbers and a valance band refers to the "energy levels in an atom where the electrons that participate in bonding occupy. These energy levels correspond to those of the s and p orbitals of the outermost shell of the atom being considered." Hope this helps!

What is the term used to label the energy levels of electrons
Energy Level Diagram - Different Energy Shells Around the Nucleus - BYJUS Electrons of an atom occupying particular orbitals have a particular energy. This is called energy level. When an electron alleviates from a high energy state to a lower energy state, emission of light occurs. What is an electron shell? The space around the nucleus which is filled will force more electrons out from the nucleus. quizlet.com › 163763201 › chapter-5-chem-flash-cardsChapter 5 Chem Flashcards | Quizlet Quantum of energy is the amount of energy required to Farther the higher the electron the blank it is from the nucleus Atomic Orbital often thought of as a region of space in which there is a high probability of finding an electron Principle quantum numbers the term used to label the energy levels of electrons S used to denotate a spherical orbital Electron Configuration - Detailed Explanation, Filling of orbital ... The Aufbau principle: electrons must completely fill the atomic orbitals of a given energy level before occupying an orbital associated with a higher energy level. Electrons occupy orbitals in the increasing order of orbital energy level. Pauli's exclusion principle: states that no two electrons can have equal values for all four quantum numbers.
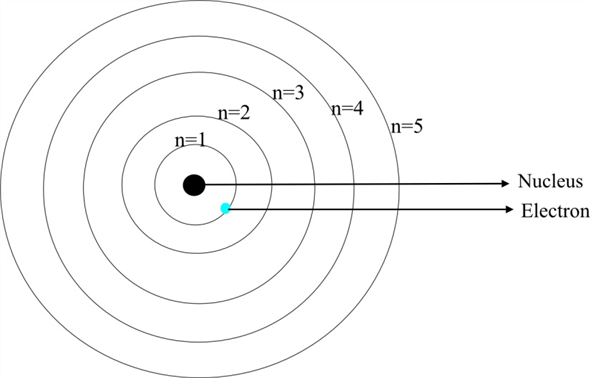
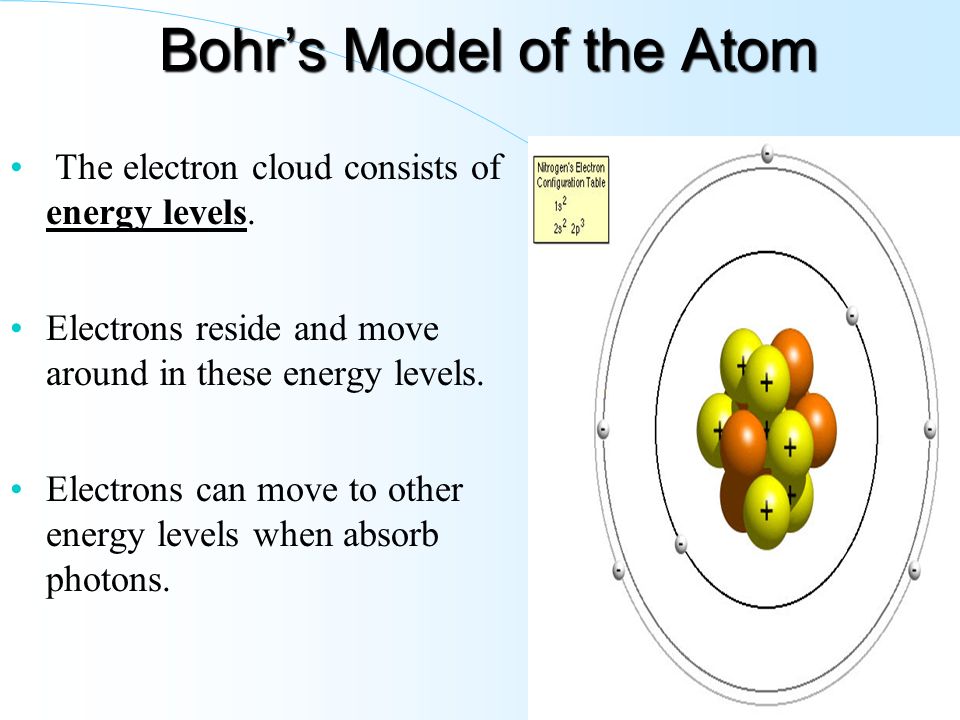
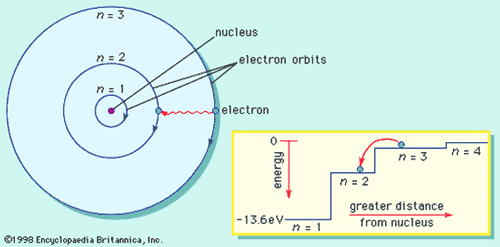
What is the term used to label the energy levels of electrons. The Periodic Table and Energy-Level Models - Middle School Chemistry The electrons surrounding an atom are located in regions around the nucleus called "energy levels". An energy level represents the 3-dimensional space surrounding the nucleus where electrons are most likely to be. The first energy level is closest to the nucleus. The second energy level is a little farther away than the first. Energy Level: Definition, Equation (w/ Diagrams) | Sciencing The outermost energy level of an atom is called the valence energy level. The electrons in this energy level are involved in any interaction the atom has with other atoms. If the energy level is full (two electrons for an s orbital, six for a p orbital and so on), then the atom isn't likely to react with other elements. What is the term used to label the energy levels of electron Answer: The quantum mechanical model of the atom estimates the probability of finding an electron in a certain position. ... Circle the letter of the formula for the maximum number of electrons that can accupy a principal energy level. Use n for the principal quantum number. Advertisement Still have questions? Find more answers Ask your question Atom - Orbits and energy levels | Britannica In quantum mechanics each orbiting electron is represented by a mathematical expression known as a wave function —something like a vibrating guitar string laid out along the path of the electron's orbit. These waveforms are called orbitals. See also quantum mechanics: Bohr's theory of the atom. Electron shells
What term is used to label energy levels of electrons? - Answers What term is used to label energy levels of electrons? Wiki User. ∙ 2014-03-14 00:39:22. Study now. See answer (1) Best Answer. Copy. Principal quantum numbers (n). Florine Wiza ∙ . Lvl 13. Energy Levels in the Periodic Table | Sciencing The s-orbital is always the first to be filled in each energy level. The first two columns of the periodic table are known as the s-block. This means that the valence electrons for these two columns exist in an s-orbital. The first energy level only contains an s-orbital. For example, hydrogen has one electron in the s-orbital. quizlet.com › 56870384 › chapter-5-test-section-51Chapter 5 test Section 5.1 Flashcards | Quizlet The term that is used to label the energy levels of electrons is? S What letter is used to denote a spherical orbital? Dumbell All "p" orbital's are _____ shaped? 2n^2 What is the formula for the maximum number of electrons that can occupy a principal energy level? (Use "n" for the principal quantum number) Sets with similar terms What term is used to label the energy levels of electrons? - BRAINLY Answer : The term used to label the energy levels of electrons is, Principle Quantum Numbers (n). Explanation : There are 4 quantum number. Principle Quantum Numbers : It describes the size of the orbital or energy levels of electrons. It is represented by n. n = 1,2,3,4.... Azimuthal Quantum Number : It describes the shape of the orbital.
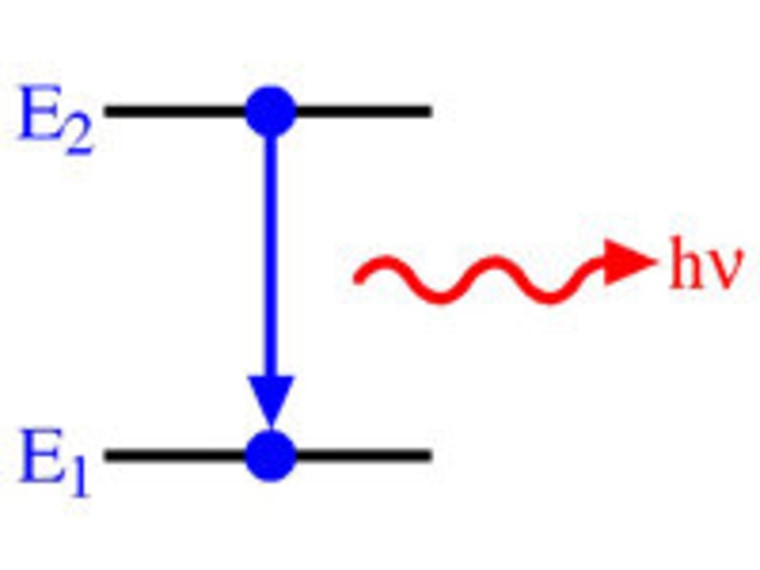
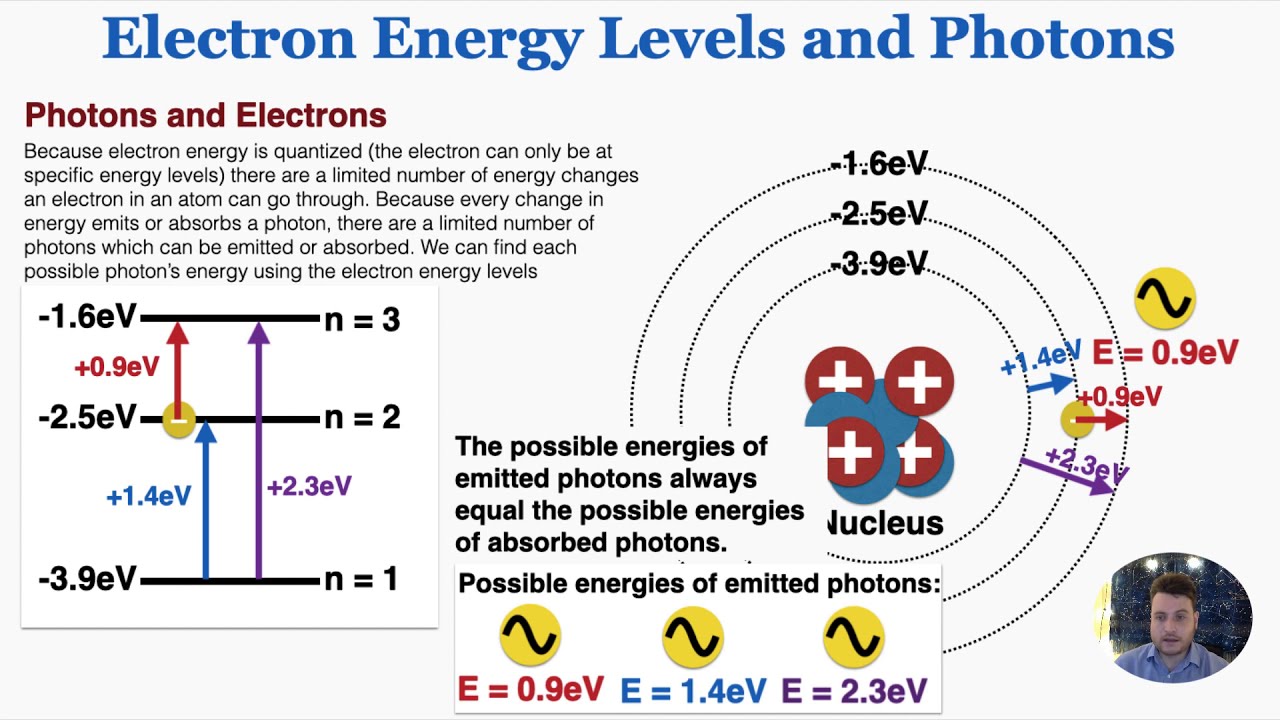
Electron Test Practice ANSWERS.doc - Electron Practice... When an electron falls from a higher energy level to a lower energy level, how isthe energy released? In a packet of energy called a PHOTON. The wavelength it istraveling in gives us colors in the visible spectrum (ROYGBV) 3. The further the electron is from the nucleus, the ____ HIGHER__ energy theelectron has. 4. A (n) _ orbital › v › atomic-energy-levelsAtomic Energy Levels (video) | Khan Academy In order to find the energy of the photon that was absorbed or emitted, you always take the higher energy level and subtract from it the lower energy level. So in this case, we would take -6eV, and subtract from it -10eV, which tells us that it would take a four eV photon to bump an electron up to that energy level, and the electron would emit a four eV photon if it dropped back down from that level. How to Represent Electrons in an Energy Level Diagram Chemists use the energy level diagram as well as electron configuration notation to represent which energy level, subshell, and orbital are occupied by electrons in any particular atom. Chemists use this information in these ways: To predict what type of bonding will occur with a particular element and show exactly which electrons are being used quizlet.com › 331198828 › chapter-5-electrons-inChapter 5 Electrons in Atoms Flashcards | Quizlet What are the fixed energies of electrons called? energy levels Circle the letter of the term that completes the sentence correctly. A quantum of energy is the amount of energy required to a. place an electron in an energy level. b. maintain an electron in its present energy level. c. move an electron from its present energy level to a higher one. C
Definition of Principal Energy Level - ThoughtCo Updated on July 14, 2019. In chemistry, the principal energy level of an electron refers to the shell or orbital in which the electron is located relative to the atom's nucleus. This level is denoted by the principal quantum number n. The first element in a period of the periodic table introduces a new principal energy level.
What is the term used to label the energy levels of electrons? What term is used to label energy levels of electrons? Principal quantum numbers (n). What is the term valance band? It refers to the energy levels in an atom where the...
Energy Level and Transition of Electrons - Brilliant In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. According to Bohr's theory, electrons of an atom revolve around the nucleus on certain orbits, or electron shells. Each orbit has its specific energy level, which is expressed as a negative value. This is because the electrons on the orbit are " ...
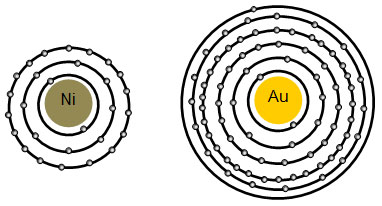
The periodic table, electron shells, and orbitals - Khan Academy The number of electrons in the outermost shell of a particular atom determines its reactivity, or tendency to form chemical bonds with other atoms. This outermost shell is known as the valence shell, and the electrons found in it are called valence electrons.
Electron Configuration - Detailed Explanation, Filling of orbital ... The Aufbau principle: electrons must completely fill the atomic orbitals of a given energy level before occupying an orbital associated with a higher energy level. Electrons occupy orbitals in the increasing order of orbital energy level. Pauli's exclusion principle: states that no two electrons can have equal values for all four quantum numbers.
quizlet.com › 163763201 › chapter-5-chem-flash-cardsChapter 5 Chem Flashcards | Quizlet Quantum of energy is the amount of energy required to Farther the higher the electron the blank it is from the nucleus Atomic Orbital often thought of as a region of space in which there is a high probability of finding an electron Principle quantum numbers the term used to label the energy levels of electrons S used to denotate a spherical orbital
Energy Level Diagram - Different Energy Shells Around the Nucleus - BYJUS Electrons of an atom occupying particular orbitals have a particular energy. This is called energy level. When an electron alleviates from a high energy state to a lower energy state, emission of light occurs. What is an electron shell? The space around the nucleus which is filled will force more electrons out from the nucleus.




















:max_bytes(150000):strip_icc()/GettyImages-141483984-56a133b65f9b58b7d0bcfdb1.jpg)

Post a Comment for "39 what is the term used to label the energy levels of electrons"